The page is in process.
The complex chromosome cycle in Acricotopus and the Orthochladiinae
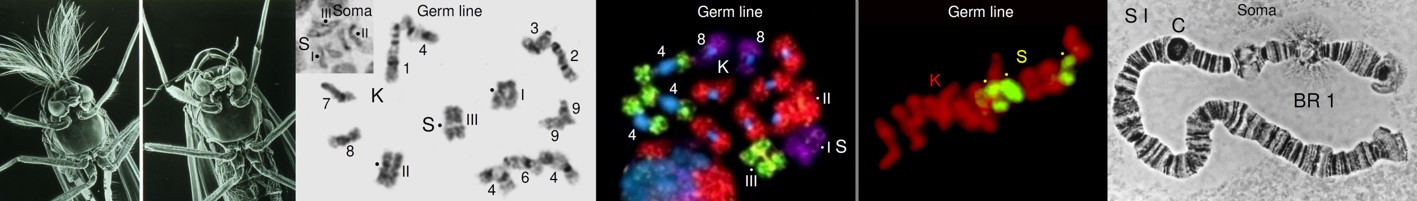
Chromosomes limited to the germ line and eliminated from the future somatic cells during early embryonic development were found in Acricotopus lucidus, a member of the Orthocladiinae, a subfamily of the Chironomidae (Diptera; Bauer and Beermann 1952).
In the Orthocladiinae these germ line-limited chromosomes (=Ks, K derived from ‘Keimbahn’, Bauer 1970) and the soma chromosomes (=Ss) pass through a complex chromosome cycle which exhibits three remarkable specialities (Fig.1; for review, see Beermann 1956; White 1973):
1 The elimination of all Ks from the prospective somatic nuclei during the early syncytial divisions (soma elimination). The Ks remain in the equatorial zone, while the soma chromosomes (=Ss) segregate regularly.
2 The elimination of about half of the Ks during the first gonial mitoses of the primordial germ cells in newly hatched larvae (germ line elimination, Bauer and Beermann, 1952).
3 A compensating duplication of the Ks as a result of the last gonial mitosis in the young fourth instar larvae. In this so-called differential mitosis all Ks move undivided to one cell pole, whereas the Ss behave as in a normal mitosis. The cells with Ss and Ks develop into regular spermatocytes and oocytes, but the cells with the S set only differentiate into aberrant spermatocytes or into nurse cells.
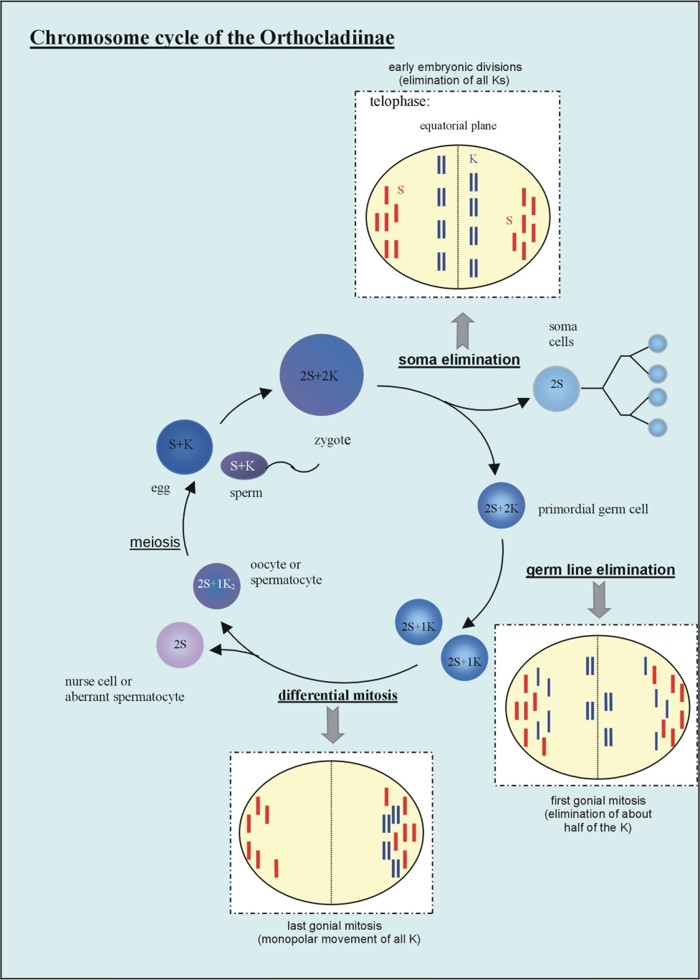
Fig. 1. In Acricotopus lucidus the soma chromosomes (=S, 2n=6, red) pass through a complex chromosome cycle together with the germ line-limited chromosomes (=K, n=6-16, blue). 1S, 2S, 1K, 2K = one or two sets of Ss or Ks, 1K2 = one set of unseparated sister chromatids.
Most relevant chromosomal events in male differential gonial mitosis, meiosis I and meiosis II of Acricotopus lucidus
A characteristic feature of the last gonial mitosis before meiosis in Acricotopus lucidus is the migration of all Ks as unseparated sister chromatids to only one pole of the cell, as shown in the spermatogonial differential mitoses in Fig. 2.
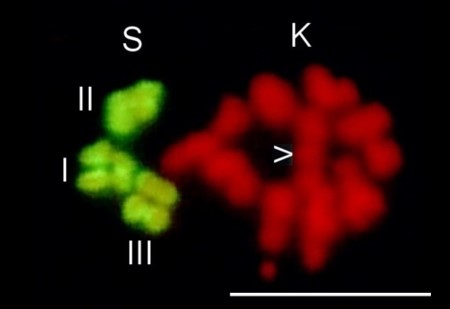
Fig. 2. Differential spermatogonial mitosis. The K migrate undivided to the right cell pole.
S, soma chromosomes; K, germ lime-limited chromosomes. I-III, SI-SIII. Bar 10 µm.
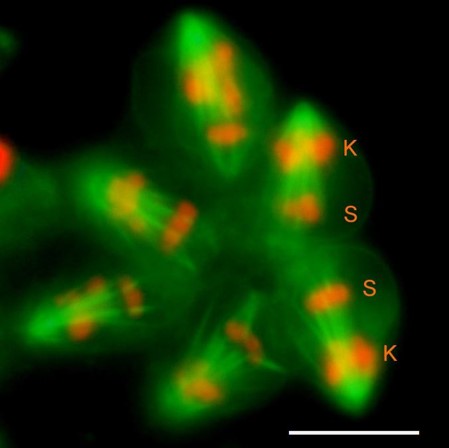
Fig. 3. Asymmetric spindles in differential mitoses. Microtubules, green; chromosomes, red. S, soma chromosomes; K, germ lime-limited chromosomes. Bar 10 µm
In contrast, the Ss (2n=6) first remain at the metaphase plate as three groups of paired homologues (Fig. 2, Fig. 3). Then, just before the Ks arrive at one spindle pole, the chromatids separate and are partitioned equally to the daughter cells (Fig. 4).
In the male, the cell receiving Ss and all the Ks develops into a primary spermatocyte (sp I, Fig. 5), which undergoes meiosis (Fig.6), whereas the other cell with only Ss follows the developmental pathway of an aberrant spermatocyte (asp, Fig. 5). The cytokinesis after this differential mitosis is incomplete, and the primary and the aberrant spermatocyte remain connected by a permanent cytoplasmic canal (Fig. 5, Fig. 6) (Staiber 2007).
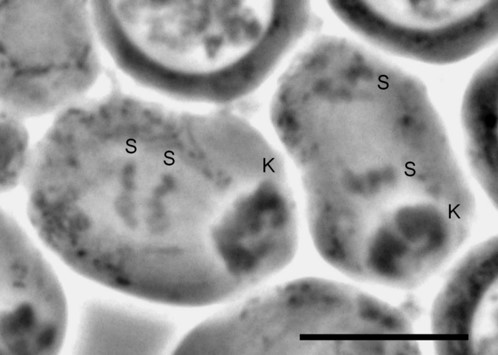
Fig. 4. Two living spermatogonia in the differential mitosis. The Ss have separated and migrate to the opposite cell poles. Bar 10 µm.
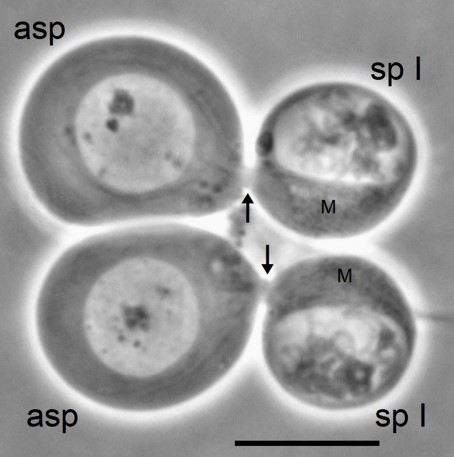
Fig. 5. Two complexes of primary spermatocyte (sp I) and aberrant spermatocyte (asp). Cytoplasmic canals (arrows). M, mitochondria. Bar 10 µm.
When the primary spermatocyte enters meiosis I, the Ss in the connected aberrant spermatocyte undergo chromosome condensation but the aberrant spermatocyte remains undivided, with the condensed metaphase status and inactivation of the Ss persisting during both meiotic divisions (Fig. 6) (Staiber 2008).

Fig. 6 Diagram summarising the most relevant chromosomal events in male differential gonial mitosis, meiosis I and meiosis II of Acricotopus lucidus. To clarify interpretation, both chromatids are represented in the condensed chromosomes. S, soma chromosomes (red; 2n=6); K, germline-limited chromosomes (blue; variable number); asp, aberrant spermatocyte; sp I, primary spermatocyte; sp II, secondary spermatocyte; sp, spermatid.
These events indicate a programmed inactivation of all chromosomes in the aberrant spermatocyte at the beginning of meiosis. The inactivation of the Ss is suggested to have developed during evolution to inhibit the entry of the aberrant spermatocytes into meiosis, thereby preventing the formation of sperms containing only Ss but no Ks (Staiber 2016).
References
Bauer, H (1970) Rearrangements between germ-line limited and somatic chromosomes in Smittia parthenogenetica (Chironomidae, Diptera). Chromosoma 32:1-10
Bauer, H, Beermann, W (1952) Der Chromosomencyclus der Orthocladiinen (Nematocera, Diptera). Z Naturforsch 7b:557-563
Beermann, W (1956) Nuclear differentiation and functional morphology of chromosomes. Cold Spring Harbor Symp Quant Biol 21:217-230
Staiber W (2007). Asymmetric distribution of mitochondria and of spindle microtubules in opposite directions in differential mitosis of germ line cells in Acricotopus. Cell Tissue Res 329:197–203
Staiber W (2008) Centrosome hyperamplification with the formation of multiple asters and programmed chromosome inactivation in aberrant spermatocytes during male meiosis in Acricotopus. Cell Tissue Res 334:81-91
Staiber, W (2016) Loss of centromeric histone H2AT120 phosphorylation accompanies somatic chromosomes inactivation in the aberrant spermatocytes of Acricotopus lucidus (Diptera, Chironomidae). Protoplasma 253:211-216
White, MJD (1973) Animal cytology and evolution, 3rd edn, Cambridge University Press, Cambridge. pp 500-546